Medications play a crucial role in treating a wide range of health conditions, from minor ailments to serious diseases like cancer and heart failure. While we may think of medications as being designed for specific purposes, many drugs have the potential to address multiple conditions simultaneously. This practice of prescribing a drug for a use other than what it was originally approved for is known as off-label drug use. In this discussion, we will delve into the realm of off-label drug prescriptions to shed light on what they entail and why they are integral to modern healthcare.
In the United States, all medications must undergo approval from the Food and Drug Administration (FDA) before they can be marketed for specific uses. Once a drug receives FDA approval, healthcare providers can prescribe it for those approved uses. When a physician prescribes a drug for a purpose not listed on its label, such as treating a different condition or using a different dosage, it is considered off-label use. This practice, which accounts for a significant portion of prescriptions, is a common and legal aspect of medical care.
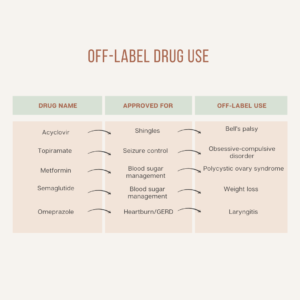
Here is a refined table displaying examples of common off-label drug use:
Legal Aspects of Off-Label Prescriptions
Off-label drug prescriptions are entirely legal in the U.S., as the FDA focuses on approving medications for their initial intended uses but does not regulate or prohibit off-label prescribing. Healthcare providers, including physicians, nurse practitioners, and pharmacists, have the authority to use their clinical judgment to prescribe medications off-label when it is in the best interest of their patients. While the FDA does not oversee off-label prescribing, pharmaceutical companies are restricted from promoting drugs for unapproved uses.
When considering off-label drug use, patient safety remains paramount. Healthcare providers carefully assess factors such as the patient’s medical history, existing medications, and potential interactions before prescribing a drug off-label. While all medications carry risks, off-label use is supported by medical evidence and professional expertise. Many off-label uses have been extensively researched and are backed by clinical data, making them a safe and effective treatment option when overseen by a healthcare provider.
Off-label drug prescriptions are a vital component of modern medical practice, offering patients access to treatments that may not have approved alternatives. While there are risks associated with any medical intervention, off-label drug use, when appropriately prescribed, can provide valuable therapeutic options. Patients should communicate openly with their healthcare providers about any concerns or unexpected side effects to ensure safe and effective treatment.